Abstract
Introduction: Forodesine is a novel inhibitor of purine nucleoside phosphorylase (PNP), which catalyzes the conversion of deoxyguanosine and guanosine to their respective bases. Inhibition of PNP leads to nucleotide pool imbalances, thereby resulting in selective apoptosis of T-cells. Recent evidence indicates that specific types of nuclear acids, including guanosine and its derivatives, act as natural ligands for toll-like receptor 7 (TLR7). We therefore speculate that the use of PNP inhibitors during inflammatory conditions may sustain considerable levels of guanine nucleosides released from damaged tissues, leading to activation of innate immunity and effector T-cell function through TLR7 activation.
Methods: To evaluate the effect of forodesine on T-cells in vivo, we used our previously described xenogeneic mouse model of GVHD, since mouse T-cells are considerably less sensitive to forodesine than human T-cells. After human T-cell transfer, mice were treated with forodesine (20 mg/kg) or vehicle daily for two weeks. Mice were monitored daily and weighed three times a week for up to 60 days after transplantation. The severity of GVHD was assessed based on a clinical GVHD scoring system.
Results: Although the mice that received human T-cells exhibited severe GVHD soon after transplantation, both guanosine and deoxyguanosine were hardly detected in the plasma, suggesting that purine nucleosides released from damaged tissues were rapidly degraded by the action of PNP. Supporting this idea, inhibition of PNP activity by forodesine markedly increased plasma guanosine levels, while deoxyguanosine was still undetectable. This was also the case in GVHD target organs, including liver and lungs.
At the same level of guanosine in the liver, forodesine promoted TNF-a production from in vitro alveolar macrophage cultures. Preincubation with a TLR7 antagonist, ODN20958, attenuated the TNF-a production from macrophages stimulated by guanosine and forodesine. Nuclease treatment did not reduce macrophage TNF-a production, suggesting that single-strand RNA, a well-known TLR7 ligand, is not involved in this response. Similar results were observed in Flt3-ligand-induced plasmacytoid DCs. Not only TNF-a but also IL-12 production was promoted by the combination of guanosine and forodesine, and was comparable to that in pDCs pulsed with R848, a potent agonist of TLR7/8. These observations suggest that a sustained high level of guanosine caused by forodesine treatment leads to continuous activation of TLR7 signaling, resulting in cytokine production from APCs. Meanwhile, as reported previously, forodesine inhibited the proliferation of human T-cells activated with CD3 and CD28 antibodies in the presence of deoxyguanosine. Of note, however, this combination did not impair but rather stimulated IFN-g production from activated T-cells cocultured with pDCs. This suggests that T-cell priming capacity mediated by the interaction with pDCs is augmented through TLR7 activation.
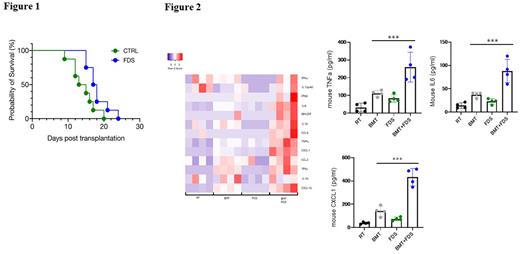
Finally, we investigated the in vivo effects of forodesine on survival, body weight loss, and disease severity of GVHD mice, and their cytokine responses. Forodesine treatment significantly prolonged survival of the mice compared with control mice, with a median survival of 17.5 days vs 14 days, respectively (p = 0.029) (Figure 1). The mean body weight loss percentage at day 9 was 11.5% vs 19.0% in the forodesine group and control, respectively (p = 0.003). Furthermore, the clinical GVHD scores were also lower in the forodesine group vs control (0.6 vs 4.9 points, p = 0.001). Consistent with our in vitro experiments, forodesine-treated mice showed significantly higher levels of serum inflammatory cytokines, such as TNF-a and IL-6 (p < 0.001). Chemokine ligands, including CXCL1, CXCL10 and CCL5, were also elevated in the mice treated with forodesine, again suggesting in vivo upregulation of TLR7 signaling pathway (Figure 2).
Conclusion: This study demonstrates that forodesine induces the selective T-cell death and ameliorates GVHD phenotype in xenogeneic GVHD mice, while amplifies host innate immune response through TLR7 signaling.
Disclosures
Ikeda:Mundipharma Pharmaceuticals: Research Funding. Sato:Mundipharma Pharmaceuticals: Research Funding. Kanda:Mundipharma Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal